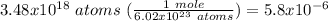Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:00
What is the mass of 2.00 l of an intravenous glucose solution with a density of 1.15 g/ml?
Answers: 2

Chemistry, 22.06.2019 14:30
Calculate the mass of carbon in 97.0 g of sucrose c12h22o11
Answers: 3

Chemistry, 22.06.2019 17:40
Areaction in which products can react to re-form reactants is
Answers: 1

Chemistry, 22.06.2019 19:10
How does the atmosphere to make earth livable? check all that apply. causes the seasons contains oxygen provides warmth creates important nutrients blocks harmful energy from the sun plz like !
Answers: 2
You know the right answer?
Which equivalence factor should you use to convert from 3.48 x 1018 atoms of mg to moles of mg?
Questions

Mathematics, 13.07.2019 02:30

Mathematics, 13.07.2019 02:30

Mathematics, 13.07.2019 02:30



History, 13.07.2019 02:30



History, 13.07.2019 02:30



Mathematics, 13.07.2019 02:30

Mathematics, 13.07.2019 02:30


Biology, 13.07.2019 02:30


Physics, 13.07.2019 02:30

English, 13.07.2019 02:30