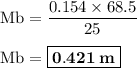Answers: 1


Another question on Chemistry

Chemistry, 20.06.2019 18:04
4moles of nitrogen gas are confined to a 6.0 l vessel at 177 °c and 12.0 atm. if the vessel is allowed to expand isothermally to 36.0 l, what would be the final pressure?
Answers: 3

Chemistry, 22.06.2019 04:00
Asolution contains 225 g of sodium chloride, nacl, dissolved in enough water to make a 0.25 l of solution. what is the molarity of the solution?
Answers: 2

Chemistry, 22.06.2019 05:20
Asolution contains 180 g of glucose (c6h12o6) and 162 g of water. what is the mole fraction of glucose?
Answers: 3

Chemistry, 22.06.2019 07:30
In the particles are arranged in a regular, repeating pattern. a)a crystalline liquid b)a crystalline solid c)all gases d)all solids
Answers: 2
You know the right answer?
By titration it is found that 68.5 ml of 0.154 m naoh(aq) is needed to neutralize 25.0 ml of hcl(aq)...
Questions


Mathematics, 20.11.2019 08:31



History, 20.11.2019 08:31




Physics, 20.11.2019 08:31

Mathematics, 20.11.2019 08:31

Mathematics, 20.11.2019 08:31


Computers and Technology, 20.11.2019 08:31



History, 20.11.2019 08:31


Biology, 20.11.2019 08:31

Biology, 20.11.2019 08:31