Consider the nuclear equation below.
what is the nuclide symbol of x?
a) 23...

Chemistry, 29.12.2019 16:31 treytonmesser
Consider the nuclear equation below.
what is the nuclide symbol of x?
a) 231/94 pu
b) 235/90 th
c) 239/94 pu
d) 231/90 th
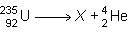

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:50
Using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 pb+2(aq) + 2cl -(aq). if the value of ksp was determined to be only 1.2 x 10-2: too much solid has dissolved. additional precipitate is forming. the solution is unsaturated. the ions are now combining to reduce their concentrations.
Answers: 3

Chemistry, 22.06.2019 10:30
Find the number of grams of hcl needed to react completely with .50 moles of magnesium.
Answers: 1

Chemistry, 22.06.2019 12:00
Hey guys so i need to know what is _nh3+> nh4oh ~chemistry~
Answers: 1

Chemistry, 22.06.2019 13:30
How many protons, electrons, and neutrons are in each of the following isotopes? a. zirconium-90 b. palladium-108 c. bromine-81 d. antimony-123
Answers: 1
You know the right answer?
Questions


English, 18.12.2020 18:40

Mathematics, 18.12.2020 18:40


Arts, 18.12.2020 18:40


Arts, 18.12.2020 18:40

Mathematics, 18.12.2020 18:40

Mathematics, 18.12.2020 18:40

English, 18.12.2020 18:40



Mathematics, 18.12.2020 18:40


English, 18.12.2020 18:40

Geography, 18.12.2020 18:40

Mathematics, 18.12.2020 18:40

Mathematics, 18.12.2020 18:40


History, 18.12.2020 18:40



