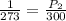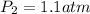Neon gas occupies a metal container of 5.5 l at stp (273 k, 1 atm). the volume of the metal container cannot be changed. what will happen to the pressure if the temperature rises to 300k? a. it will fall to 0.7 atm. b. it will fall to 0.9 atm. c. it will rise to 1.1 atm. d. it will rise to 1.3 atm.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Temperature and kinetic energy are proportional. a) adirectly b) directly c) indirectly
Answers: 2


Chemistry, 22.06.2019 02:00
What is the maximum number of electrons that an atomic orbital can contain?
Answers: 1

You know the right answer?
Neon gas occupies a metal container of 5.5 l at stp (273 k, 1 atm). the volume of the metal containe...
Questions





Social Studies, 03.12.2021 01:00


Mathematics, 03.12.2021 01:00


Computers and Technology, 03.12.2021 01:00


Chemistry, 03.12.2021 01:00


Mathematics, 03.12.2021 01:00

Physics, 03.12.2021 01:00

Computers and Technology, 03.12.2021 01:00

Chemistry, 03.12.2021 01:00




English, 03.12.2021 01:00

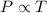 (At constant volume and number of moles)
(At constant volume and number of moles)
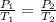
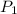 = initial pressure of gas = 1 atm
= initial pressure of gas = 1 atm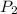 = final pressure of gas = ?
= final pressure of gas = ?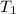 = initial temperature of gas =
= initial temperature of gas = 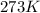
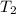 = final temperature of gas = 300 K
= final temperature of gas = 300 K