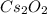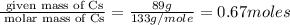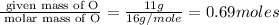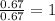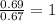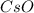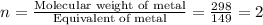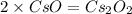Chemistry, 11.11.2019 19:31 tesadeshazer
What is the molecular formula of a compound containing 89% cesium (cs) and 11% oxygen (o) with a molar mass = 298 g/mol?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:20
Which formula can be used to calculate the molar mass of ammonia (nh3)? molar mass of n + molar mass of h 3 × molar mass of n + molar mass of h molar mass of n + 3 × molar mass of h 3 × molar mass of n + 3 × molar mass of h
Answers: 1

Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 3

Chemistry, 23.06.2019 01:00
Which process results in the release of energy stored in the products of photosynthesis? a. polymer synthesis b. depolymerization c. digestion d. cellular respiration
Answers: 1

Chemistry, 23.06.2019 04:00
Silver reacts with oxygen to produce silver oxide. (write balanced chemical equation and identify type of chemical reaction.)
Answers: 1
You know the right answer?
What is the molecular formula of a compound containing 89% cesium (cs) and 11% oxygen (o) with a mol...
Questions




Mathematics, 22.04.2020 01:52

Mathematics, 22.04.2020 01:52


Mathematics, 22.04.2020 01:52


Mathematics, 22.04.2020 01:52




Mathematics, 22.04.2020 01:52




Mathematics, 22.04.2020 01:52


Mathematics, 22.04.2020 01:52