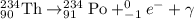Chemistry, 17.09.2019 17:50 ayoismeisalex
Fiestaware is radioactive because the glaze used contains uranium oxide. the half-life of the uranium-238 isotope present in these dishes is about 4.5 billion years. this implies that most of the uranium-238 is still in the dishes and very little has decayed to emit ionizing radiation. uranium-238 initially decays into thorium-234 and an alpha particle as shown in the background, but thorium-234 can further decay to emit beta particles and gamma radiation. what is the decay reaction of thorium-234?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Type the correct answer in the box. spell all words correctly.what is the correct term for living the most sustainable life you can within your current circumstances? when your are being as sustainable as you can within your current lifestyle, you are said to be sustainability.
Answers: 3

Chemistry, 22.06.2019 10:30
Earth's axis of rotation is tilted at an angle of 23.5 degrees. what is one change you would see on earth if its axis was not tilted?
Answers: 3

Chemistry, 22.06.2019 15:00
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 2

Chemistry, 22.06.2019 22:30
Calculate the concentration of all species in a 0.165 m solution of h2co3.
Answers: 1
You know the right answer?
Fiestaware is radioactive because the glaze used contains uranium oxide. the half-life of the uraniu...
Questions

Mathematics, 29.11.2019 23:31


Chemistry, 29.11.2019 23:31


Social Studies, 29.11.2019 23:31

Mathematics, 29.11.2019 23:31


Social Studies, 29.11.2019 23:31

Mathematics, 29.11.2019 23:31


History, 29.11.2019 23:31








Mathematics, 29.11.2019 23:31