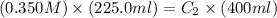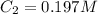Chemistry, 24.12.2019 19:31 odymonster9
Initially a beaker contains 225.0 ml of a 0.350 m mgso4 solution. then 175.0 ml of water are added to the beaker. find the concentration of the final solution. what is the volume?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:40
1) in saturated limewater, [h+ ]=3.98x10-13 m. a) find [oh]-/ b) what is the ph? / c) is the solution acidic, basic, or neutral? / 2) in butter, [h+ ]=6.0x10-7 m. a) find [oh]-/ b) what is the ph? / c) is the solution acidic, basic, or neutral? / 3) in peaches, [oh]=3.16x10-11 m a) find [h+ ]/ b) what is the ph? / c) is the solution acidic, basic, or neutral? / 4) during the course of the day, human saliva varies between being acidic and basic. if [oh]=3.16x10-8 m, a) find [h+ ]/ b) what is the ph? / c) is the solution acidic, basic, or neutral? /
Answers: 3

Chemistry, 22.06.2019 14:30
Which of the following describes a situation where competition between producers exists
Answers: 1

Chemistry, 22.06.2019 14:50
How are evaporation and sublimation similar? a both involve the formation of a gas. b both release energy to the surroundings. c both take place throughout a solid. d both take place at the surface of a liquid.
Answers: 1

Chemistry, 22.06.2019 18:00
The fact that the total amount of energy in a system remains constant is a(n)
Answers: 1
You know the right answer?
Initially a beaker contains 225.0 ml of a 0.350 m mgso4 solution. then 175.0 ml of water are added t...
Questions


Social Studies, 02.10.2019 20:00

Health, 02.10.2019 20:00


Mathematics, 02.10.2019 20:00

Mathematics, 02.10.2019 20:00


Mathematics, 02.10.2019 20:00

History, 02.10.2019 20:00

Biology, 02.10.2019 20:00

Mathematics, 02.10.2019 20:00

Advanced Placement (AP), 02.10.2019 20:00





Social Studies, 02.10.2019 20:00

Computers and Technology, 02.10.2019 20:00


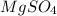 + Volume of water added
+ Volume of water added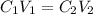
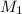 = concentration of
= concentration of 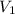 = volume of
= volume of 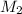 = concentration of final solution = ?
= concentration of final solution = ?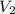 = volume of final solution = 400 ml
= volume of final solution = 400 ml