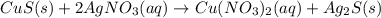Chemistry, 15.11.2019 20:31 Tringirl233
E. solid copper sulfide and silver nitrate react to form copper (ii) nitrate and solid silver sulfide. write a balanced chemical equation that describes the reaction. identify the oxidation number of each element in the reaction. (you do not need to include the total contribution of charge.) is this reaction a redox reaction or a non-redox reaction? explain your answer.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 13:50
How do the energy differences between the higher energy levels of an atom compare with the energy difference between the lower energy level of the atom
Answers: 1


Chemistry, 22.06.2019 10:30
What woukd most likely be the transmittance at a 0.70 m solution of solute a? a) 7.6%b) 1.1%c)4.0%d)4.6%
Answers: 1

Chemistry, 22.06.2019 11:30
Aperfume bottle is dropped in the corner of a room. the odor of the perfume can be detected on the other side of the room. which statement best describes this observation?
Answers: 2
You know the right answer?
E. solid copper sulfide and silver nitrate react to form copper (ii) nitrate and solid silver sulfid...
Questions


Mathematics, 02.04.2020 01:59



Mathematics, 02.04.2020 01:59

English, 02.04.2020 01:59

Mathematics, 02.04.2020 01:59



Mathematics, 02.04.2020 01:59


Mathematics, 02.04.2020 01:59

Mathematics, 02.04.2020 01:59


English, 02.04.2020 01:59

Mathematics, 02.04.2020 01:59


Business, 02.04.2020 01:59


Chemistry, 02.04.2020 01:59