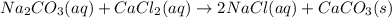Which represents a double replacement reaction and would occur?
a) nh4no3(aq) + agcio3(aq) mc...

Chemistry, 21.09.2019 12:30 ismailhajisaid29101
Which represents a double replacement reaction and would occur?
a) nh4no3(aq) + agcio3(aq) mc011-1.jpg agno3(aq) + nh4cio3(aq)
b) na2co3(aq) + cacl2(aq) mc011-2.jpg 2nacl(aq) + caco3(s)
c) naf(aq) + kcl(aq) mc011-3.jpg nacl(aq) + kcl(aq)
d) nacl(aq) mc011-4.jpg na+(aq) + cl-(aq)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 19:30
Phosphorous can form an ion called phosphide, which has the formula p3−. this ion can form an ion called phosphide, which has the formula p3−. this ion properties very similar to those of pforms when a phosphorus atom loses three protonsis called a cationcontains 18 electrons
Answers: 2

Chemistry, 23.06.2019 00:30
When a beta particle is emitted, the mass number of the nucleus a. decreases by one b. increases by one c. remains the same d. decreases by two
Answers: 2

Chemistry, 23.06.2019 06:30
How can the number of core electrons be determined from the periodic table
Answers: 1

Chemistry, 23.06.2019 17:30
What mineral is this? color: varies streak: colorless indistinct/fracture luster: non fluoresces
Answers: 1
You know the right answer?
Questions





Biology, 25.10.2019 22:43

Physics, 25.10.2019 22:43

Biology, 25.10.2019 22:43

Mathematics, 25.10.2019 22:43

Mathematics, 25.10.2019 22:43

Health, 25.10.2019 22:43

Advanced Placement (AP), 25.10.2019 22:43







Computers and Technology, 25.10.2019 22:43