
Chemistry, 23.09.2019 05:30 montgomerykarloxc24x
Neon has an average atomic mass of 20.2 g/mol, whereas argon has an average atomic mass of 40.0 g/mol. how would the number of atoms in a 1.0 mol sample of neon compare to the number of atoms in a 4.0 mol sample of argon

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:20
Which of these statements explains the difference between nuclear binding energy and the strong nuclear force ?
Answers: 3

Chemistry, 22.06.2019 11:30
What is the main reason why some developing countries fear the increase the free trade policies around the world?
Answers: 2

Chemistry, 22.06.2019 12:20
Adeuteron, 21h, is the nucleus of a hydrogen isotope and consists of one proton and one neutron. the plasma of deuterons in a nuclear fusion reactor must be heated to about 3.02×108 k . what is the rms speed of the deuterons? express your answer using two significant figures.
Answers: 1

You know the right answer?
Neon has an average atomic mass of 20.2 g/mol, whereas argon has an average atomic mass of 40.0 g/mo...
Questions




Mathematics, 17.12.2020 14:00


English, 17.12.2020 14:00



Spanish, 17.12.2020 14:00





Physics, 17.12.2020 14:00

Mathematics, 17.12.2020 14:00




Mathematics, 17.12.2020 14:00

Mathematics, 17.12.2020 14:00

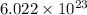 number of atoms.
number of atoms.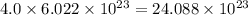 number of atoms
number of atoms

