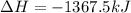Chemistry, 22.11.2019 08:31 aprilreneeclaroxob0c
Using the standard enthalpies of formation found in the textbook, determine the enthalpy change for the combustion of ethanol c2h5oh as given below. c2h5oh (l) + 3 o2(g) → 2 co2(g) + 3 h2o(g)

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Which type of reaction always has an element and a compound as reactants
Answers: 1

Chemistry, 22.06.2019 08:30
Draw the skeletal structures of two different molecules that are each made of 5 carbon atoms and 12 hydrogen atoms.
Answers: 1

Chemistry, 22.06.2019 10:00
In a water molecule, hydrogen and oxygen are held together by a(an) bond. a) double covalent b) ionic c) nonpolar covalent d) hydrogen e) polar covalent
Answers: 1

Chemistry, 22.06.2019 11:30
Aperfume bottle is dropped in the corner of a room. the odor of the perfume can be detected on the other side of the room. which statement best describes this observation?
Answers: 2
You know the right answer?
Using the standard enthalpies of formation found in the textbook, determine the enthalpy change for...
Questions

Mathematics, 03.12.2019 00:31



Mathematics, 03.12.2019 00:31

History, 03.12.2019 00:31


Mathematics, 03.12.2019 00:31








Geography, 03.12.2019 00:31


English, 03.12.2019 00:31

Mathematics, 03.12.2019 00:31

Mathematics, 03.12.2019 00:31

Mathematics, 03.12.2019 00:31

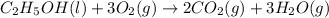
![\Delta H=\sum [n\times H_f(product)]-\sum [n\times H_f(reactant)]](/tpl/images/0386/1954/c8099.png)
![\Delta H=[(n_{CO_2}\times H_f_{CO_2})+(n_{H_2O}\times H_f_{H_2O}) ]-[(n_{O_2}\times H_f_{O_2})+(n_{C_2H_5OH}\times H_f_{C_2H_5OH})]](/tpl/images/0386/1954/91c50.png)
![\Delta H=[(2\times -393.5 kJ/mol)+(3\times -285.5 k) ]-[(3\times 0)+(1\times -276]](/tpl/images/0386/1954/80382.png)