
Chemistry, 03.10.2019 12:20 krystalhurst97
The hydrogen atoms of a water molecule are bonded to the oxygen atom by bonds, whereas neighboring water molecules are held together by bonds. 79)
a. polar covalent; ionic
b. hydrogen; polar covalent
c. ionic; covalent
d. polar covalent; hydrogen

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
If 10.g of agno3 is available, what volume of 0.25 m agno3 can be prepared
Answers: 1

Chemistry, 22.06.2019 04:00
Acontainer holds 35.8 moles of gas under 10.0 atm of pressure at 70.0 c. what is the volume of the container?
Answers: 2

Chemistry, 22.06.2019 08:40
For each of the following compounds, write the formula then predict whether it would be a strong, weak, or non-electrolyte when placed in di water. for the ionic compounds only, put (s) or (aq) after the forrmula formula strong, weak or non electrolyte? a calcium hydroxide b. silver carbonate c. lead(ii) sulfate d. phosphorus trifluoride e. sodium phosphide f barium sulfate g. strontium acetate h. zinc nitrate
Answers: 3

Chemistry, 22.06.2019 10:30
If you add 5.00 ml of 0.100 m sodium hydroxide to 50.0 ml of acetate buffer that is 0.100 m in both acetic acid and sodium acetate, what is the ph of the resulting solution? acetic acid: ka = 1.8. x 10-5
Answers: 1
You know the right answer?
The hydrogen atoms of a water molecule are bonded to the oxygen atom by bonds, whereas neighboring...
Questions

Biology, 13.10.2020 06:01

History, 13.10.2020 06:01






Chemistry, 13.10.2020 06:01

Mathematics, 13.10.2020 06:01

Biology, 13.10.2020 06:01

English, 13.10.2020 06:01

Mathematics, 13.10.2020 06:01


Mathematics, 13.10.2020 06:01

Biology, 13.10.2020 06:01


Biology, 13.10.2020 06:01


Social Studies, 13.10.2020 06:01

Mathematics, 13.10.2020 06:01

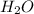 . In water molecules, two hydrogen atoms are bonded to oxygen atom by two covalent bonds. Covalent bond is formed by sharing of electrons. As oxygen atom is more electronegative than hydrogen, therefore, shared electrons are attracted towards oxygen atoms. Because of this covalent bonds between hydrogen and oxygen are polar in nature.
. In water molecules, two hydrogen atoms are bonded to oxygen atom by two covalent bonds. Covalent bond is formed by sharing of electrons. As oxygen atom is more electronegative than hydrogen, therefore, shared electrons are attracted towards oxygen atoms. Because of this covalent bonds between hydrogen and oxygen are polar in nature.

