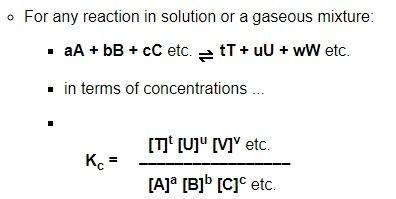1. which processes are taking place in the system represented by the following equation?
2co(...

1. which processes are taking place in the system represented by the following equation?
2co(g) + o2(g) →← 2co2(g)
2. consider the reaction represented by the equation 2hi(g) →← h2(g) + i2(g). at a temperature of 520 °c, the equilibrium concentration of hi is 0.80 m, of h2 is 0.010 m, and of i2 is 0.010 m. what is the k for the reaction?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:40
For a patient with the following pes statement and interventions, which would be the most appropriate monitoring and evaluating data? pes statement: inadequate calcium intake related to food and nutrition related knowledge deficit as evidenced by statements that the only dietary source of calcium is milk and she believes that she is lactose intolerant. patient’s nutrition prescription is for a diet providing 1200 mg calcium per day. patient was provided with in-depth nutrition education on alternative dietary and supplement sources of calcium. a. calcium intake (at subsequent visit) b. knowledge assessment by asking patient to identify food sources from menus and shopping list (at the end of the current visit) c. serum calcium (at next visit) d. both a and b e. both a and c
Answers: 2

Chemistry, 22.06.2019 03:00
Compare the valence electron configuration of the nobles gas elements seen here. what statement is correct?
Answers: 2

Chemistry, 22.06.2019 09:00
Particles vibrate in a rigid structure and do not move relative to their neighbors.
Answers: 1

Chemistry, 22.06.2019 12:00
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al oxidizing agent = reducing agent =
Answers: 1
You know the right answer?
Questions

Mathematics, 09.04.2021 01:00

Mathematics, 09.04.2021 01:00


History, 09.04.2021 01:00


Mathematics, 09.04.2021 01:00

History, 09.04.2021 01:00




History, 09.04.2021 01:00

English, 09.04.2021 01:00

Mathematics, 09.04.2021 01:00


Advanced Placement (AP), 09.04.2021 01:00

Mathematics, 09.04.2021 01:00

Mathematics, 09.04.2021 01:00


Mathematics, 09.04.2021 01:00

History, 09.04.2021 01:00