What is the ∆g for the following reaction under standard conditions (t = 298 k) for the formation of nh4no3(s)? 2nh3(g) + 2o2(g) nh4no3(s) + h2o(l) given: nh4no3(s): ∆hf = -365.56 kj ∆sf = 151.08 j/k. nh3(g): ∆hf = -46.11 kj ∆sf = 192.45 j/k. h2o(l): ∆hf = -285.830 kj ∆sf = 69.91 j/k. o2(g): ∆hf = 0.00 kj ∆sf = 205 j/k. 186.6 kj 6.9 kj -10.4 kj -126.3 kj -382 kj

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:00
If the density of water is 1.0 g/cm3, which of these materials would float in water, based on their densities? check all that apply. aluminum cork iron lead wax
Answers: 1

Chemistry, 22.06.2019 06:30
Suppose a lab group reports a ppercent yield of sand of 105. is it really possible to collect more sand than was originally represented? what is the possible explanation for the extra product?
Answers: 2

Chemistry, 22.06.2019 16:10
Given the following equation: 2a1 + 3mgcl2 --> 2alcl3 + 3mg how many moles of aluminum chloride are produced from 2.5 moles of magnesium chloride?
Answers: 1

Chemistry, 23.06.2019 01:40
Calcium carbonate decomposes at high temperatures to give calcium oxide and carbon dioxide as shown below. caco3(s) cao(s) + co2(g) the kp for this reaction is 1.16 at 800°c. a 5.00 l vessel containing 10.0 g of caco3(s) was evacuated to remove the air, sealed, and then heated to 800°c. ignoring the volume occupied by the solid, what will be the mass of the solid in the vessel once equilibrium is reached?
Answers: 1
You know the right answer?
What is the ∆g for the following reaction under standard conditions (t = 298 k) for the formation of...
Questions







Mathematics, 22.10.2020 22:01

Mathematics, 22.10.2020 22:01

English, 22.10.2020 22:01





English, 22.10.2020 22:01

English, 22.10.2020 22:01

English, 22.10.2020 22:01

Mathematics, 22.10.2020 22:01


English, 22.10.2020 22:01

Computers and Technology, 22.10.2020 22:01

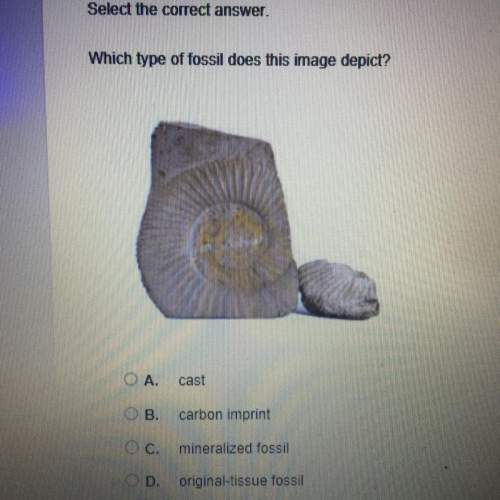
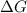 for the reaction is -382 kJ.
for the reaction is -382 kJ.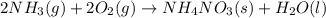
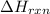 is:
is: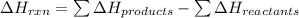
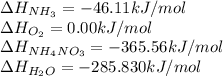
![\Delta H_{rxn}=[1(\Delta H_{NH_4NO_3})+1(\Delta H_{H_2O})]-[2(\Delta H_{NH_3})+2(\Delta H_{O_2})]](/tpl/images/0477/9423/fc8bd.png)
![\Delta H_{rxn}=[1(-365.56)+1(-285.83)]-[2(-46.11)+2(0)]kJ\\\\\Delta H_{rxn}=-559.17kJ=559170J](/tpl/images/0477/9423/fe79f.png)
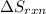 is:
is: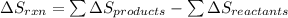
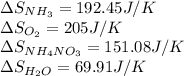
![\Delta S_{rxn}=[1(\Delta S_{NH_4NO_3})+1(\Delta S_{H_2O})]-[2(\Delta S_{NH_3})+2(\Delta S_{O_2})]](/tpl/images/0477/9423/3dbc2.png)
![\Delta S_{rxn}=[1(151.08)+1(69.91)]-[2(192.45)+2(205)]J/K\\\\\Delta S_{rxn}=-573.91J/K](/tpl/images/0477/9423/e9201.png)
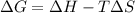
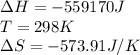
![\Delta G=(-559170J)-[298K\times (-573.91J/K)]\\\\\Delta G=-382kJ](/tpl/images/0477/9423/ce61d.png)