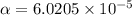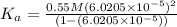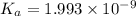Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Alarge marble is dropped in a graduated cylinder with 35ml of water in it.the water level increases to 49ml.what is the volume of the marble
Answers: 1

Chemistry, 22.06.2019 01:30
Reaction rate depends on how many molecules are coming into contact with each other with enough energy to react. increasing the temperature of the reactants will increase -
Answers: 3

Chemistry, 22.06.2019 03:40
In an effort to address concerns about global warming, a power plant in portland,oregon is designed to take all of its exhaust gases from its boilers and recycle the co2 using the solvay process to make sodium hydrogen carbonate. the reaction is shown below. nh3(g) + h2o(l) + co2(g) + nacl(aq) → nahco3(aq) + nh4cl(aq) how many liters each of nh3 and co2 (both at stp) would be consumed to produce 3.00 kg of sodium bicarbonate? the volume of both nh3 and co2 would be
Answers: 1

Chemistry, 22.06.2019 11:10
Which of the following shapes would represent a molecule with two bonded atoms and 3 lone pairs on only one of them , trigonal planar , bent , trigonal pyramidal , linear
Answers: 1
You know the right answer?
The ph of a 0.55 m aqueous solution of hypobromous acid, hbro, at 25.0°c is 4.48. what is the value...
Questions

Mathematics, 28.03.2021 14:00

English, 28.03.2021 14:00



English, 28.03.2021 14:00

Health, 28.03.2021 14:00

Mathematics, 28.03.2021 14:00

Chemistry, 28.03.2021 14:00

Social Studies, 28.03.2021 14:00


Social Studies, 28.03.2021 14:00


English, 28.03.2021 14:00


Geography, 28.03.2021 14:00





 .
.![[HBrO]=c=0.55 M](/tpl/images/0481/5864/42f9d.png)
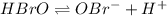
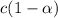
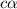
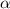 .
.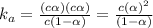 ..(1)
..(1)![[H^+]=c\alpha](/tpl/images/0481/5864/9b43d.png)
![pH=4.48=-\log[H^+]=-\log[c\alpha ]=-\log[0.55 M\times \alpha ]](/tpl/images/0481/5864/f198d.png)