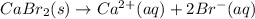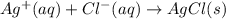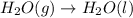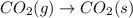Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 13:40
Can someone me with 6 to 10 plz this is for masteries test.
Answers: 1


Chemistry, 23.06.2019 01:30
Ascientist is measuring the pressure that is exerted by each of the following gases in the atmosphere: carbon dioxide, oxygen, and nitrogen. which term most likely describes what she is measuring?
Answers: 1

Chemistry, 23.06.2019 10:00
How many moles are equal to 2.4×10^23 formula units of sodium chloride
Answers: 1
You know the right answer?
For which of the following processes would you expect there to be an increase in entropy? ag+(aq) +...
Questions



Mathematics, 12.07.2021 23:10

Biology, 12.07.2021 23:10


Biology, 12.07.2021 23:10

History, 12.07.2021 23:10

English, 12.07.2021 23:10

Mathematics, 12.07.2021 23:10

Arts, 12.07.2021 23:10






Spanish, 12.07.2021 23:20

Mathematics, 12.07.2021 23:20

Mathematics, 12.07.2021 23:20


Mathematics, 12.07.2021 23:20