Asample of dolomitic limestone containing only caco3 and mgco3 was analyzed.
a. when a 0.2800...

Chemistry, 30.08.2019 17:40 paolaviviana
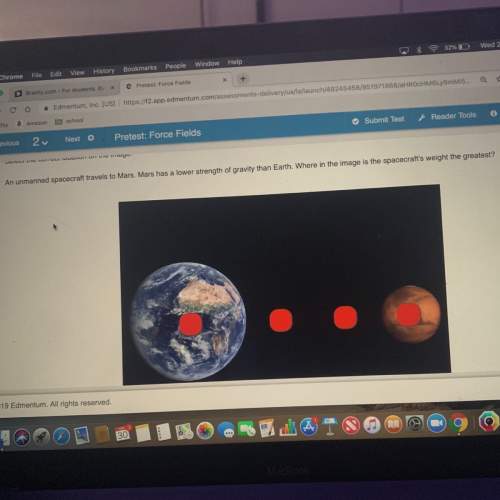
Asample of dolomitic limestone containing only caco3 and mgco3 was analyzed.
a. when a 0.2800 gram sample of this limestone was decomposed by heating, 75.0 milliliters of co2 at 750 mmhg and 20 degrees celcius were evovled. how many grams of co2 were produced.
b. write the equations for the decomposition of both carbonates described above.
c. it was also determined that the initial sample contained 0.0488 gram of calcium. what percent of the limestone by mass was caco3?
d. how many grams of the magnesium- containing product were present in the sample in (a) after it had been heated?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:00
This classification of drug typically changes the brain's chemistry and reduces its ability to create its own endorphins.
Answers: 1

Chemistry, 22.06.2019 09:00
What is the percentage composition of carbon in the compound ch4
Answers: 1

Chemistry, 22.06.2019 14:30
Ahypothesis must be testable and falsifiable to be considered scientific a. trueb. false
Answers: 1

You know the right answer?
Questions



Mathematics, 30.10.2020 02:40



Mathematics, 30.10.2020 02:40






Mathematics, 30.10.2020 02:40



Advanced Placement (AP), 30.10.2020 02:40


History, 30.10.2020 02:40


English, 30.10.2020 02:40

Chemistry, 30.10.2020 02:40