
Chemistry, 31.08.2019 13:00 coolkiddKC
Ga sealed container holds 0.020 moles of nitrogen (n2) gas, at a pressure of 1.5 atmospheres and a temperature of 290 k. the atomic mass of nitrogen is 14.0 g/mol. the boltzmann constant is 1.38 × 10-23 j/k and the ideal gas constant is r = 8.314 j/ mol · k = 0.0821 l · atm/mol · k. the mass density of the gas is closest to

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:00
6. using 3 – 4 sentences explain (in your own words) why water expands when it freezes? 7. using your knowledge of colligative properties explain whether sodium chloride or calcium chloride would be a more effective substance to melt the ice on a slick sidewalk. use 3 – 4 sentences in your explanation.
Answers: 1



Chemistry, 23.06.2019 05:30
Elizabeth has two separate samples of the same substance. sample is in the liquid state, and the other is in the solid state. the two samples most likely differ in which property?
Answers: 1
You know the right answer?
Ga sealed container holds 0.020 moles of nitrogen (n2) gas, at a pressure of 1.5 atmospheres and a t...
Questions


Physics, 04.07.2019 20:00



English, 04.07.2019 20:00

Physics, 04.07.2019 20:00

Mathematics, 04.07.2019 20:00




English, 04.07.2019 20:00


Mathematics, 04.07.2019 20:00



Mathematics, 04.07.2019 20:00


Mathematics, 04.07.2019 20:00


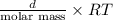
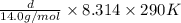
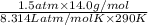
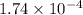 g
g

