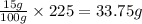Chemistry, 01.09.2019 11:30 hannah2757
Potassium sulfate has a solubility of 15g/100g water at 40 celsius. a solution is prepared by adding 39.0g of potassium sulfate to 225g water, carefully heating the solution, and cooling it to 40 celsius. a homogeneous solution is obtained. is this solution saturated, unsaturated, or supersaturated? the beaker is shaken and precipitation occurs. how many grams of potassium sulfate would you except to crystallize out?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Sex cells from female reproductive organ? 1) mitosis 2) fertilization 3) zygote 4) eggs 5) meiosis 6) sperm
Answers: 2

Chemistry, 22.06.2019 09:00
Scientific evidence tells us that the cause of earths four season is the tilt of earth as it revolves around the sun. the student is instructed to illustrate this information in a science notebook. how will the student illiterate winter in the northern hemisphere?
Answers: 3


Chemistry, 22.06.2019 21:30
Which of the following changes will decrease the total amount of gaseous solute able to be dissolved in a liter of liquid water? (2 points) decreasing temperature decreasing pressure decreasing surface area decreasing solute concentration
Answers: 1
You know the right answer?
Potassium sulfate has a solubility of 15g/100g water at 40 celsius. a solution is prepared by adding...
Questions


Mathematics, 07.12.2020 20:00

History, 07.12.2020 20:00

Biology, 07.12.2020 20:00


Chemistry, 07.12.2020 20:00



Mathematics, 07.12.2020 20:00

English, 07.12.2020 20:00


Chemistry, 07.12.2020 20:00






Arts, 07.12.2020 20:00