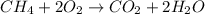Chemistry, 11.10.2019 10:50 rebecca7415
Methane (ch4) is the main component of natural gas. it is burned for fuel in a combustion reaction. the unbalanced combustion reaction for methane is shown below. ch4 + o2 co2 + h2o + heat when the reaction is balanced, how many carbon dioxide molecules are produced for every methane molecule burned?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:20
What would you do if you told the guy you liked that you liked him
Answers: 1


Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 22.06.2019 13:00
Asubstance is a good conductor of electricity which of the following best explains a probable position of the substance in a periodic table
Answers: 3
You know the right answer?
Methane (ch4) is the main component of natural gas. it is burned for fuel in a combustion reaction....
Questions




Computers and Technology, 15.07.2020 02:01

Computers and Technology, 15.07.2020 02:01

Mathematics, 15.07.2020 02:01

Mathematics, 15.07.2020 02:01

Mathematics, 15.07.2020 02:01

Mathematics, 15.07.2020 02:01

Computers and Technology, 15.07.2020 02:01



Mathematics, 15.07.2020 02:01

Mathematics, 15.07.2020 02:01

Mathematics, 15.07.2020 02:01


Mathematics, 15.07.2020 02:01