
Chemistry, 23.09.2019 14:20 demetriascott20
Iron ore is reduced to pure iron by smelting, during which the iron (iii) oxide in the ore reacts with carbon monoxide gas, like this: fe2o3s + 3cog → 2fes + 3co2g suppose an engineer decides to study the rate of this reaction. she prepares four reaction vessels with 158.8g of solid iron (iii) oxide and 33.9g of carbon monoxide gas each. the volume and temperature of each vessel is shown in the table below. arrange the reaction vessels in decreasing order of initial rate of reaction. in other words, select a "1" next to the vessel in which the engineer can reasonably expect the initial rate of reaction to be highest, a "2" next to the vessel in which the initial rate of reaction would be next highest, and so on. rate of reaction a 2.0l 1100.°c b 2.0l 1200.°c c 4.0l 1100.°c d 4.0l 1000.°c

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 12:30
Achemist determined by measurements that 0.0300 most of beryllium oxide participate in a chemical reaction calculate the mass of berlylium oxide that participates
Answers: 3


Chemistry, 22.06.2019 07:10
Which of these conditions most likely produces an unstable isotope?
Answers: 2

Chemistry, 22.06.2019 21:30
In science class richard learns that a substance has a boiling point of 230 fahrenheit his teacher ask him to convert this temperature to degrees celsius what is the boiling point of his substance in degrees celsius
Answers: 3
You know the right answer?
Iron ore is reduced to pure iron by smelting, during which the iron (iii) oxide in the ore reacts wi...
Questions

Mathematics, 24.09.2019 21:10

English, 24.09.2019 21:10



English, 24.09.2019 21:10



History, 24.09.2019 21:10





History, 24.09.2019 21:10




Business, 24.09.2019 21:10

Mathematics, 24.09.2019 21:10

Mathematics, 24.09.2019 21:10

Mathematics, 24.09.2019 21:10

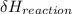 = negative.
= negative.

