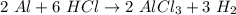Chemistry, 20.08.2019 23:50 liyahheadhigh
Which of the following products would be possible in the reaction of 2 moles of aluminum (al) with 6 moles of hydrochloric acid (hcl)? a. 2 moles of alcl2 and 1 mole of h2 b. 3 moles of alcl3 and 2 moles of h2 c. 2 moles of alcl3 and 3 moles of h2 d. 3 moles of alcl2 and 3 moles of h2

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:50
If a reactant was removed, did the new equilibrium system shift to make more reactants or more products?
Answers: 1

Chemistry, 22.06.2019 23:00
What is the average rate of the reaction between 10 and 20 s?
Answers: 1

Chemistry, 23.06.2019 00:00
The empirical formula of a compound is ch2o and its mass is 120 amu/molecule, what is its formula?
Answers: 1

Chemistry, 23.06.2019 01:30
Select the correct answer from each drop-down menu. to make a table of the elements, dmitri mendeleev sorted the elements according to their . he then split the list of elements into several columns so that elements beside each other had similar .
Answers: 2
You know the right answer?
Which of the following products would be possible in the reaction of 2 moles of aluminum (al) with 6...
Questions

History, 17.11.2020 21:30

Mathematics, 17.11.2020 21:30

Health, 17.11.2020 21:30

English, 17.11.2020 21:30



English, 17.11.2020 21:30





Mathematics, 17.11.2020 21:30


Mathematics, 17.11.2020 21:30


Mathematics, 17.11.2020 21:30

Physics, 17.11.2020 21:30

English, 17.11.2020 21:30

Mathematics, 17.11.2020 21:30