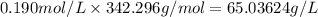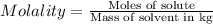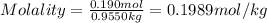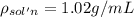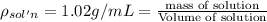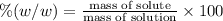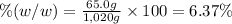Chemistry, 02.10.2019 12:30 irenecupcake4348
Asucrose solution is prepared to a final concentration of 0.190 m . convert this value into terms of g/l, molality, and mass % (molecular weight, mwsucrose = 342.296 g/mol ; density, ρsol′n = 1.02 g/ml ; mass of water, mwat = 955.0 g ). note that the mass of solute is included in the density of the solution.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:00
Endeleev saw trends in the physical and chemical properties of elements when he organized them by
Answers: 2

Chemistry, 23.06.2019 05:00
Match each term to its description. match term definition excess reactant a) reactant that can produce a lesser amount of the product limiting reactant b) amount of product predicted to be produced by the given reactants theoretical yield c) reactant that can produce more of the product
Answers: 3

Chemistry, 23.06.2019 10:40
Question 17 hydrogen is manufactured on an industrial scale by this sequence of reactions: +ch4gh2og ⇌ +cog3h2g k1 +cogh2og ⇌ +co2gh2g k2 the net reaction is: +ch4g2h2og ⇌ +co2g4h2g k write an equation that gives the overall equilibrium constant k in terms of the equilibrium constants k1 and k2. if you need to include any physical constants, be sure you use their standard symbols, which you'll find in the aleks calculator.
Answers: 2

Chemistry, 23.06.2019 17:10
Two changes are described below. a green banana turns yellow and ripens. a layer of rust forms on an iron nail. which statement is true about the two changes? a) both are chemical changes because new substances are formed. b) both are physical changes because only the physical state of the substances change. c) a is a physical change due to a change of state, but b is a chemical change because new molecules are formed. d) a is a chemical change due to a change of state, but b is a physical change because new molecules are formed.
Answers: 1
You know the right answer?
Asucrose solution is prepared to a final concentration of 0.190 m . convert this value into terms of...
Questions

Chemistry, 12.10.2019 13:10

Mathematics, 12.10.2019 13:10

Mathematics, 12.10.2019 13:10


History, 12.10.2019 13:10

English, 12.10.2019 13:10

English, 12.10.2019 13:10

Biology, 12.10.2019 13:10

English, 12.10.2019 13:10


Mathematics, 12.10.2019 13:10

Social Studies, 12.10.2019 13:10

Advanced Placement (AP), 12.10.2019 13:10

Mathematics, 12.10.2019 13:10

Mathematics, 12.10.2019 13:10

Biology, 12.10.2019 13:10



World Languages, 12.10.2019 13:10

History, 12.10.2019 13:10