
Chemistry, 01.09.2019 02:30 nulledcracker12
Consider the balanced chemical equation that follows. you are asked to determine how many moles of water you can produce from 4.0 mol of hydrogen and excess oxygen. (excess oxygen means that so much oxygen is available it will not run out.) which of the numbers that appear in the balanced chemical equation below are used to perform this calculation? 2h2(g)+o2(g)→2h2o(l)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:30
In this chemical reaction, 325 grams of barium (ba) react completely. how many moles of lithium (li) are produced?
Answers: 1

Chemistry, 22.06.2019 04:00
What layer of the atmosphere is directly above the troposphere?
Answers: 1

Chemistry, 22.06.2019 04:20
Which of the following is true for the actual yield of a reaction? it is always calculated as a ratio. it is the yield from the excess reactant. it is the yield from the limiting reactant. it is always less than the theoretical yield.
Answers: 1

Chemistry, 22.06.2019 05:30
Astudent carefully transfers 30 g of water and 30 g of alcohol in a glass tube, forming two layers and filling the tube completely. after sealing the tube, the student mixes the solutions, and notices a bubble that forms in the tube. what is the mass of the contents in the glass tube after mixing?
Answers: 2
You know the right answer?
Consider the balanced chemical equation that follows. you are asked to determine how many moles of w...
Questions


Mathematics, 06.05.2020 22:07

Mathematics, 06.05.2020 22:07

Mathematics, 06.05.2020 22:07



History, 06.05.2020 22:07


Mathematics, 06.05.2020 22:07

Mathematics, 06.05.2020 22:07

Mathematics, 06.05.2020 22:07


History, 06.05.2020 22:07

Advanced Placement (AP), 06.05.2020 22:07


History, 06.05.2020 22:07


Mathematics, 06.05.2020 22:07

Physics, 06.05.2020 22:07

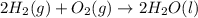
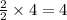 moles of water
moles of water

