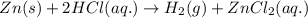Chemistry, 24.08.2019 05:20 caprisun1491
A1.0-gram strip of zinc is reacted with hydrochloric acid in a test tube. the unbalanced equation below represents the reaction.
zn(s) + hcl(aq) ==> h2(g) + zncl2(aq)
balance the equation in your answer booklet for the reaction of zinc and hydrochloric acid, using the smallest whole-number coefficients. [1]
zn(s) + hcl(aq) ==> h2(g) + zncl2(aq)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:30
What volume of a 2.00 m stock solution of naoh is needed to prepare 150. ml of 0.40 m solution?
Answers: 2

Chemistry, 21.06.2019 18:30
Which orbitals form a pi bond? a.the s orbital and three p orbitals b.the s orbital and two p orbitals c.overlapping p orbitals d.overlapping hybrid orbitals
Answers: 2

Chemistry, 22.06.2019 00:10
Think about how you can use le chatelier’s principle to find possible solutions to the design problem. describe at least two ways to increase the yield (amount) of ammonia based on this principle.
Answers: 2

Chemistry, 22.06.2019 10:00
3. how much energy in joules is required to evaporate .0005 kg of liquid ammonia to vapor at the same temperature? 4. how much energy ( in megajoules ) is given up by .75 kg of water at 0c when it freezes to form ice at 0c? 5. explain how heat works between and at critical temperatures?
Answers: 2
You know the right answer?
A1.0-gram strip of zinc is reacted with hydrochloric acid in a test tube. the unbalanced equation be...
Questions

Health, 20.11.2019 01:31



Mathematics, 20.11.2019 01:31

Mathematics, 20.11.2019 01:31




Chemistry, 20.11.2019 01:31

Mathematics, 20.11.2019 01:31

History, 20.11.2019 01:31


English, 20.11.2019 01:31





Social Studies, 20.11.2019 01:31