Which statement explains why ozone gas, o3, and oxygen gas, o2, have different properties?
(1...

Chemistry, 30.09.2019 10:30 sharnisefrazier
Which statement explains why ozone gas, o3, and oxygen gas, o2, have different properties?
(1) they are formed from different elements.
(2) they have different molecular structures.
(3) they have different oxidation numbers.
(4) they have different electronegativities.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
If 10.g of agno3 is available, what volume of 0.25 m agno3 can be prepared
Answers: 1

Chemistry, 22.06.2019 02:20
6. what does the symbol ah stand for? o one calorie given off by a reaction the specific heat of a substance the heat capacity of a substance the heat of reaction for a chemical reaction
Answers: 1

Chemistry, 22.06.2019 08:30
The mass of a neutron is equal to the mass of a proton plus the mass of an electron. true or false false true
Answers: 1

Chemistry, 22.06.2019 23:00
In which region is the substance in both the solid phase and the liquid phase? 1 2. 3 4 mark this and return save and exit next
Answers: 2
You know the right answer?
Questions




History, 05.05.2020 02:55

Mathematics, 05.05.2020 02:55

Mathematics, 05.05.2020 02:55

History, 05.05.2020 02:55

Mathematics, 05.05.2020 02:55

English, 05.05.2020 02:55

History, 05.05.2020 02:55




English, 05.05.2020 02:55

Mathematics, 05.05.2020 02:55

Mathematics, 05.05.2020 02:55

Mathematics, 05.05.2020 02:55

Mathematics, 05.05.2020 02:55

Geography, 05.05.2020 02:55

Mathematics, 05.05.2020 02:55

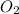 (Paramagnetic)
(Paramagnetic)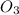 (diamagnetic)
(diamagnetic)


