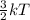Chemistry, 15.01.2020 01:31 braydentillery1221
Asample of gas is enclosed in a container of fixed volume. identify which of the following statements are true. check all that apply. if the container is heated, the gas particles will lose kinetic energy and temperature will increase.
if the container is heated, the gas particles will lose kinetic energy and temperature will increase.
if the container is cooled, the gas particles will lose kinetic energy and temperature will decrease.
if the gas particles move more quickly, they will collide more frequently with the walls of the container and pressure will increase.
if the gas particles move more quickly, they will lose energy more rapidly and collide with the walls of the container less often, and pressure will decrease.
if the gas particles move more quickly, they will collide with the walls of the container more often and with more force, and pressure will increase.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 13:30
Which of the following has wavelength longer than the wavelength of viable light? a) x rays b) gamma rays c) radios waves d) ultraviolet waves
Answers: 1

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 22.06.2019 14:00
Calculate the frequency of a wave in a spring toy. the wave has a speed of 1.1 meters per second and a wavelength of 0.1 meters. *
Answers: 2

Chemistry, 22.06.2019 16:30
Explain in detail of the four major scientific developments that spurred the formulation of the plate tectonics theory
Answers: 2
You know the right answer?
Asample of gas is enclosed in a container of fixed volume. identify which of the following statement...
Questions

English, 07.05.2021 06:20


Mathematics, 07.05.2021 06:20

Biology, 07.05.2021 06:20

Medicine, 07.05.2021 06:20


Mathematics, 07.05.2021 06:20


Mathematics, 07.05.2021 06:20


Mathematics, 07.05.2021 06:20

Medicine, 07.05.2021 06:20



Biology, 07.05.2021 06:20

World Languages, 07.05.2021 06:20

Mathematics, 07.05.2021 06:20


Physics, 07.05.2021 06:20

Biology, 07.05.2021 06:20