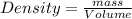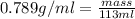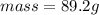Chemistry, 26.09.2019 20:30 Andrebutrus
Ethanol is a common laboratory solvent and has a density of 0.789 g/ml. what is the mass, in grams, of 113 ml of ethanol?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Using the periodic table, complete the table to describe each atom. type in your answers.a ? b? c? d? e? f?
Answers: 1

Chemistry, 22.06.2019 11:30
If blood contains 150g of hemoglobin per liter of blood, how much hemoglobin would be contained in 10 ml of blood
Answers: 2

Chemistry, 22.06.2019 20:00
The picture represents the process that produces most of the energy used by living organisms on earth. which process is represented in the picture? a) the magnetic attraction between two hydrogen nuclei. b) the fusion of hydrogen nuclei to produce a helium nucleus in the core of the sun. c) the fission of hydrogen nuclei to produce a helium nucleus in the core of the sun. d) the chemical reaction between hydrogen nuclei to produce a helium nucleus in earth's atmosphere.
Answers: 3

Chemistry, 22.06.2019 21:30
Isopropyl alcohol, (ch3)2choh, is a common solvent. determine the percent by mass of hydrogen in isopropyl alcohol. a) 6.71% h b) 13.4% h c) 25.0% h d) 53.3% h
Answers: 1
You know the right answer?
Ethanol is a common laboratory solvent and has a density of 0.789 g/ml. what is the mass, in grams,...
Questions




Business, 02.09.2020 18:01

English, 02.09.2020 18:01

Mathematics, 02.09.2020 18:01






Computers and Technology, 02.09.2020 18:01



Chemistry, 02.09.2020 18:01

Mathematics, 02.09.2020 18:01



English, 02.09.2020 18:01

Health, 02.09.2020 18:01