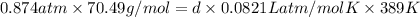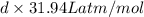Chemistry, 19.09.2019 19:00 nickname0097
What is the density (in g/l) of a gas with a molar mass of 70.49g/mol at 0.874atm and 389k?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:00
The overall chemical reaction for photosynthesis isshown below: 6co2 + 6h20 → c6h12o6 + 602what mass of glucose (c6h1206) can form from71.89 g co2? (molar mass of c6h1206 = 180.18g/mol; molar mass of co2 = 44.01 g/mol)71.89 g co2=g c6h1206
Answers: 1

Chemistry, 22.06.2019 21:30
If you burn 46.6 g of hydrogen and produce 416 g of water, how much oxygen reacted
Answers: 3

Chemistry, 22.06.2019 22:10
What is the indicator of the number of ions in solution? the amount of conductivity the amount of precipitate the amount of solute added
Answers: 1

Chemistry, 22.06.2019 22:30
Gusing the milligrams of ascorbic acid you entered above, the ratio of total sample volume to aliquot volume, and the total milligrams of the vitamin c tablet that you dissolved, calculate the mass of ascorbic acid in the vitamin c tablet for each trial. do this by scaling up to find the amount (mg) of ascorbic acid in your 250 ml flask. enter your calculated mass of ascorbic acid in the vitamin c tablet, for each trial. be sure to enter your calculated mass in the corresponding order that you entered your milligrams of ascorbic acid. the milligrams of ascorbic acid you entered for entry #1 previously should correspond to the mass of ascorbic acid that you enter for entry #1 here.
Answers: 1
You know the right answer?
What is the density (in g/l) of a gas with a molar mass of 70.49g/mol at 0.874atm and 389k?...
Questions


History, 17.10.2020 14:01


Mathematics, 17.10.2020 14:01

Mathematics, 17.10.2020 14:01





Biology, 17.10.2020 14:01


Physics, 17.10.2020 14:01

Chemistry, 17.10.2020 14:01

Mathematics, 17.10.2020 14:01

Mathematics, 17.10.2020 14:01

Mathematics, 17.10.2020 14:01



Mathematics, 17.10.2020 14:01

Mathematics, 17.10.2020 14:01

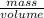
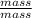
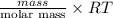
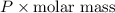 = dRT
= dRT