
Chemistry, 24.08.2019 23:20 eeromaki1321
For the reaction:
6li(s)+n2(g)→2li3n(s)
determine:
the mass of n2 needed to react with 0.536 moles of li.
the number of moles of li required to make 46.4 g of li3n.
the mass in grams of li3n produced from 3.65 g li.
the number of moles of lithium needed to react with 7.00 grams of n2.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 1

Chemistry, 22.06.2019 23:30
What are the similarities between compounds and mixtures?
Answers: 3

Chemistry, 23.06.2019 02:30
When the ionic compound nabr dissolves in water, br– ions are pulled into solution by the attraction between what two particles? a. the na+ and br– ions b. the na+ ion and the negative end of a water molecule c. the br– ion and the positive end of a water molecule d. the br– ion and the negative end of a water molecule
Answers: 1

Chemistry, 23.06.2019 02:30
What type of energy conversion occurs when you place your feet near the fire place and they become warm
Answers: 1
You know the right answer?
For the reaction:
6li(s)+n2(g)→2li3n(s)
determine:
the mass of n2 needed to reac...
6li(s)+n2(g)→2li3n(s)
determine:
the mass of n2 needed to reac...
Questions

Chemistry, 02.12.2019 18:31




History, 02.12.2019 18:31

Spanish, 02.12.2019 18:31

Mathematics, 02.12.2019 18:31

Mathematics, 02.12.2019 18:31




English, 02.12.2019 18:31



Biology, 02.12.2019 18:31

Mathematics, 02.12.2019 18:31

Mathematics, 02.12.2019 18:31

History, 02.12.2019 18:31

Mathematics, 02.12.2019 18:31

History, 02.12.2019 18:31

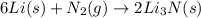
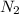 needed to react with 0.536 moles of Li.
needed to react with 0.536 moles of Li.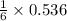 moles of
moles of ![N_2[tex] gas needed:[tex]=28 g/mol\times 0.0893 mol=2.5004 g](/tpl/images/0194/9373/6ce3e.png)
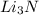
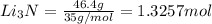
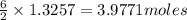
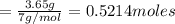
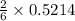 that is 0.1738 moles of
that is 0.1738 moles of 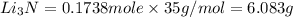
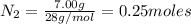
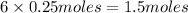 of lithium
of lithium


