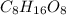Chemistry, 25.09.2019 15:00 liferoblox666
Acompound is 40.0% c, 6.70% h, and 53.3% o by mass. assume that we have a 100.-g sample of this compound. the molecular formula mass of this compound is 240 amu . what are the subscripts in the actual molecular formula?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:00
As you watch a surfer ride a wave towards the shoreline, what is the shoreline? a) displacement reference b) reference point c) coordinate plane d) cartesian boundary
Answers: 1


Chemistry, 22.06.2019 13:50
Abeaker with 2.00×102 ml of an acetic acid buffer with a ph of 5.000 is sitting on a benchtop. the total molarity of acid and conjugate base in this buffer is 0.100 m. a student adds 4.70 ml of a 0.360 m hcl solution to the beaker. how much will the ph change? the pka of acetic acid is 4.740.
Answers: 1

Chemistry, 22.06.2019 14:00
How many absorptions would you expect to observe in the 13c nmr spectra of the following molecules? a) 3-chloropentane b) cis-4-methyl-2-pentene
Answers: 2
You know the right answer?
Acompound is 40.0% c, 6.70% h, and 53.3% o by mass. assume that we have a 100.-g sample of this comp...
Questions


Computers and Technology, 29.06.2019 02:00



Mathematics, 29.06.2019 02:00

English, 29.06.2019 02:00



History, 29.06.2019 02:00

Mathematics, 29.06.2019 02:00

History, 29.06.2019 02:00






Spanish, 29.06.2019 02:00