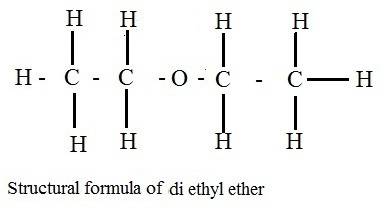Chemistry, 05.10.2019 20:00 mallardmya2006
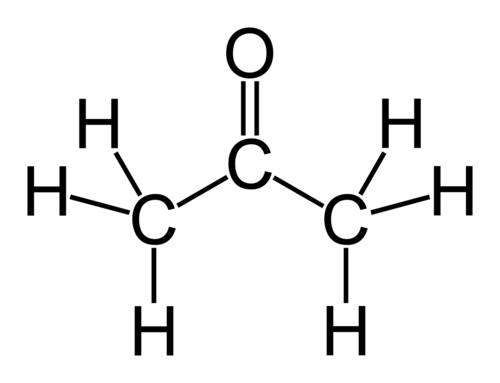
Why does ch3coch3 has stronger intermolecular forces than c2h5oc2h5? even though both have dipole-dipole as their imf, but c2h5oc2h5 has a larger molecular weight and as the molecular weight increases, the imf get stronger. so why it is the opposite here?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 2

Chemistry, 22.06.2019 04:30
How many moles of air are there in a human lung with a volume of 2.4 l at stp? explain your answer
Answers: 1

Chemistry, 22.06.2019 18:30
The number of moles of a given mass of a substance can be found without knowing its molecular formula or molar mass. true false
Answers: 1

Chemistry, 23.06.2019 00:30
Ok, so i have 2 questions. try to answer them both: (the topic is fire) 1) how can you represent the chemical reaction of fire? 2) what kind of bond is formed in this chemical reaction
Answers: 3
You know the right answer?
Why does ch3coch3 has stronger intermolecular forces than c2h5oc2h5? even though both have dipole-d...
Questions


Mathematics, 09.10.2019 23:30


Mathematics, 09.10.2019 23:30

Biology, 09.10.2019 23:30

Physics, 09.10.2019 23:30




Biology, 09.10.2019 23:30

Chemistry, 09.10.2019 23:30





Chemistry, 09.10.2019 23:30

Mathematics, 09.10.2019 23:30

Mathematics, 09.10.2019 23:30

Arts, 09.10.2019 23:30

Mathematics, 09.10.2019 23:30