Chemistry, 29.09.2019 18:30 andrejr0330jr
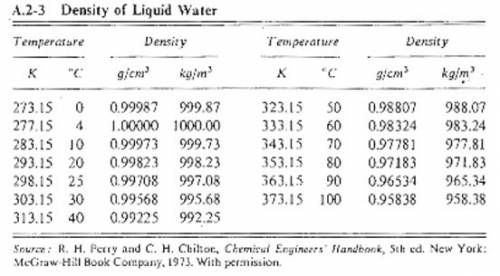
Suppose you were calibrating a 50.0 ml volumetric flask using distilled water. the flask temperature was at 20°c, and you assumed that the distilled water was as well. however, you later discover that the actual water temperature was 14°c instead. how is the mass of the 50.0 ml of distilled water you measured at 14°c different from the mass of 50.0 ml of distilled water at 20°c?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Select all that apply. a beta particle: is electromagnetic energy is an electron has zero charge is emitted from the nucleus has a +2 charge has a -1 charge
Answers: 1


Chemistry, 22.06.2019 13:30
Which statements are true concerning mineral formation? check all that apply. the slower the cooling, the larger the crystals. the faster the cooling, the smaller the crystals. crystals formed from magma are smaller than crystals formed from lava. minerals can only form in solutions when the solution is heated deep underground. when a solution cools, elements and compounds leave the solution and crystallize as minerals. minerals formed from hot water solutions can form narrow channels in the surrounding rock.
Answers: 1

Chemistry, 22.06.2019 15:30
A1.5l container holds p.50 grams of an unknown gas at a pressure of 0.44 atm and a temperature of 50.c what is the molar mass of the unknown gas
Answers: 1
You know the right answer?
Suppose you were calibrating a 50.0 ml volumetric flask using distilled water. the flask temperature...
Questions

Mathematics, 26.03.2021 08:30

Arts, 26.03.2021 08:30



Chemistry, 26.03.2021 08:30


Chemistry, 26.03.2021 08:30


Advanced Placement (AP), 26.03.2021 08:30

Chemistry, 26.03.2021 08:30

History, 26.03.2021 08:30





Arts, 26.03.2021 08:30


History, 26.03.2021 08:30