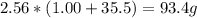2nacl + h2so4 --> 2hcl + na2so4
how many grams of hcl can be prepared from 2.00 mol h2so4...

Chemistry, 23.10.2019 03:00 julionavedo21
2nacl + h2so4 --> 2hcl + na2so4
how many grams of hcl can be prepared from 2.00 mol h2so4 and 2.56 mol nacl?
a. 7.30 g
b. 93.3 g
c. 146 g
d. 150 g
e. 196 g

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 2

Chemistry, 23.06.2019 05:30
Astudent made the lewis dot diagram of a compound as shown. mg is written with two dots shown on its top. an o is written on each side of mg. each o has six dots around it. an arrow is shown from one dot on mg toward the vacant space around the o on the right. another arrow is shown from the other dot on mg toward the vacant space around the o on the left. the title of the art is students lewis dot model. what is the error in the lewis dot diagram? an o atom should transfer all its six electrons to mg because the formula is mgo. both electrons of mg should be transferred to one o atom because the formula is mgo. the electrons should be transferred from each o atom to mg because mg has fewer electrons. the number of dots around mg should be four because it has to transfer two electrons to each o.
Answers: 2

Chemistry, 23.06.2019 09:00
How many moles of sulfur dioxide are in 2.26 × 10^33 sulfur dioxide molecules?
Answers: 3
You know the right answer?
Questions

Mathematics, 24.04.2021 05:50


Chemistry, 24.04.2021 05:50


Health, 24.04.2021 05:50



Biology, 24.04.2021 05:50

Mathematics, 24.04.2021 05:50





Mathematics, 24.04.2021 05:50


History, 24.04.2021 05:50

English, 24.04.2021 05:50

Social Studies, 24.04.2021 05:50

History, 24.04.2021 05:50

Mathematics, 24.04.2021 05:50

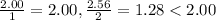 hence NaCl is the limiting reagant.
hence NaCl is the limiting reagant.