
Chemistry, 20.04.2021 04:20 chutchinson256
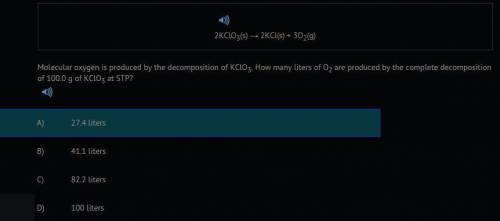
2KCLO3(s) --> 2KCL(s) + 302(g) Molecular oxygen is produced by the decomposition of KClO3. How many liters of O2 are produced by the complete decomposition of 100.0 g of KCLO3 at STP?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 20:10
Suppose you mix one mole of sulfuric acid (h2so4) with 1 mole of sodium hydroxide(naoh). why does the ph of the solution remain below 7? ( explain so i can get better understanding! )
Answers: 2

Chemistry, 22.06.2019 22:30
What must be in balance for temperatures to remain constant?
Answers: 1

Chemistry, 22.06.2019 23:00
What is the energy in joules of a mole of photons associated with visible light of wavelength 486 nm?
Answers: 3

Chemistry, 22.06.2019 23:30
Aweight lifter raises a 1600 n barbell to a height of 2.0 meters. how much work was done? w = fd a) 30 joules b) 3000 joules c) 320 joules d) 3200 joules
Answers: 2
You know the right answer?
2KCLO3(s) --> 2KCL(s) + 302(g) Molecular oxygen is produced by the decomposition of KClO3. How ma...
Questions



Mathematics, 24.04.2020 18:07


English, 24.04.2020 18:07



Advanced Placement (AP), 24.04.2020 18:07


History, 24.04.2020 18:07




Computers and Technology, 24.04.2020 18:07



History, 24.04.2020 18:07

Mathematics, 24.04.2020 18:07




