
Chemistry, 20.04.2021 09:30 zahnjoey4661
1. From the chemicals listed on your lab handout, write down the weak acid (with its pKa) and its conjugate base that would create a buffer that best fits your protein. Would you expect for your buffer to have more acid or more base?
My assigned protein is Xylanase and has an optimum pH of 5.5.
2. Buffers are used to the inhibit the change of pH upon the addition of strong acids and bases. If you were to add 0.1 M HCl to your buffer, would you expect the pH to change? If so, would the pH increase or decrease? What would happen if 0.1M NaOH were to be added instead?
3. Keeping your buffer composition from question 1 in mind, would you expect to use a larger volume of HCl or NaOH to change the pH of the buffer solution by one unit? Explain.
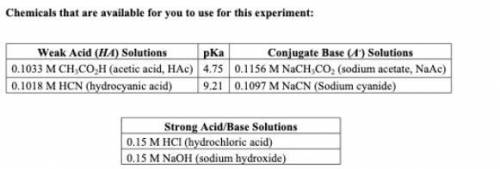

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:20
Harvey kept a balloon with a volume of 348 milliliters at 25.0˚c inside a freezer for a night. when he took it out, its new volume was 322 milliliters, but its pressure was the same. if the final temperature of the balloon is the same as the freezer’s, what is the temperature of the freezer? the temperature of the freezer is kelvins.
Answers: 2

Chemistry, 22.06.2019 02:30
Select each correct answer. more than one answer may be correct. which of the following is a characteristic of unicellular organisms? they can possess tissues and organs. all of their functions are performed by a single cell. they are usually microscopic. each of their cells is specialized to perform a specific function.
Answers: 1

Chemistry, 22.06.2019 10:00
According to the tide table below what time of day will the highest tide occur? (2 pt) the highest tide will occur at
Answers: 1

Chemistry, 22.06.2019 12:00
An atom's configuration based on its number of electrons ends at 3p4. another atom has seven more electrons. starting at 3p, what is the remaining configuration? 3p63d34s2 3p43d54s2 3p64s23d3 3p44s23d
Answers: 3
You know the right answer?
1. From the chemicals listed on your lab handout, write down the weak acid (with its pKa) and its co...
Questions

Mathematics, 04.04.2020 03:22




History, 04.04.2020 03:22



Mathematics, 04.04.2020 03:22



Mathematics, 04.04.2020 03:23

History, 04.04.2020 03:23



Mathematics, 04.04.2020 03:23


Mathematics, 04.04.2020 03:23



Mathematics, 04.04.2020 03:24



