
Chemistry, 20.04.2021 22:40 alyssaboosiefkes
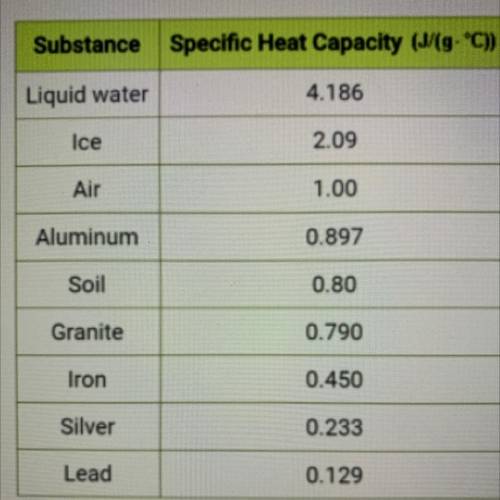
Use the specific heat value from the table at left to calculate the amount
of energy in Joules required to raise the temperature of 5.08 g of
aluminum by 13.20 °C. Do not record units in your answer. Round to
the tenths place or further.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:30
On a distance vs time graph the line of an object at rest is a
Answers: 1

Chemistry, 21.06.2019 20:30
18. use the activity series to predict whether the following synthesis reaction will occur. write the chemical equations for the reaction if it's predicted to occur. (s) + o2(g) -> *note: it is possible.*
Answers: 1

Chemistry, 22.06.2019 05:00
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change
Answers: 3

Chemistry, 22.06.2019 14:30
Is a pencil falling to the floor anon contact force, a force, or a contact force
Answers: 1
You know the right answer?
Use the specific heat value from the table at left to calculate the amount
of energy in Joules req...
Questions

Mathematics, 10.12.2021 01:50


Arts, 10.12.2021 01:50

English, 10.12.2021 02:00


Mathematics, 10.12.2021 02:00



English, 10.12.2021 02:00

Mathematics, 10.12.2021 02:00

Mathematics, 10.12.2021 02:00


Social Studies, 10.12.2021 02:00


History, 10.12.2021 02:00

Mathematics, 10.12.2021 02:00



Mathematics, 10.12.2021 02:00

Computers and Technology, 10.12.2021 02:00



