Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:50
8) a mixture of he, ne and ar has a pressure of 7.85 atm. if the ne has a mole fraction of 0.47 and 8) ar has a mole fraction of 0.23, what is the pressure of he? a) 4.2 atm b) 3.7 atm c) 5.5 atm d) 2.4 atm e) 1.8 atm
Answers: 1

Chemistry, 22.06.2019 18:40
What is the binding energy of a nucleus that has a mass defect of 5.81*10-^29 kg a 5.23*10-^12 j b 3.15* 10^12 j c 1.57*10-3 j d 9.44*10^20 j
Answers: 1

Chemistry, 23.06.2019 01:30
What is produced from neutralization of an acid and a base? a. hydronium ions b. citric acid c. salt and water
Answers: 1

Chemistry, 23.06.2019 20:30
Due tomorrow write the chemical equation that has the equilibrium constant expression [listed in photo]
Answers: 2
You know the right answer?
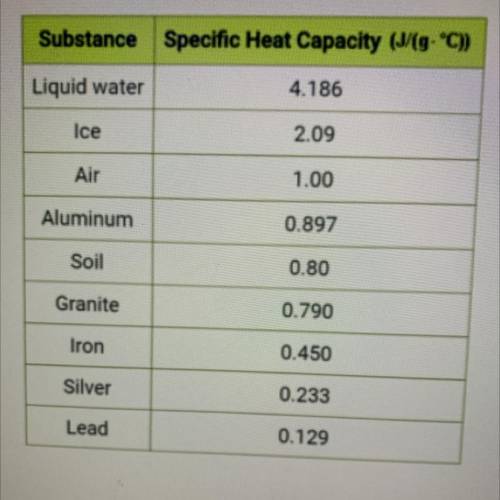
Use the specific heat value from the table at left to calculate the
temperature change in °C when...
Questions

Mathematics, 18.10.2019 06:30


Mathematics, 18.10.2019 06:30



Physics, 18.10.2019 06:30

Social Studies, 18.10.2019 06:30




Mathematics, 18.10.2019 06:30

Mathematics, 18.10.2019 06:30

Arts, 18.10.2019 06:30


Biology, 18.10.2019 06:30

English, 18.10.2019 06:30




Physics, 18.10.2019 06:30