Ok, i need a help.
Knowing that the initial concentration of phosphoric acid
within a pool i...

Chemistry, 21.04.2021 06:50 tabisulaprinces2084
Ok, i need a help.
Knowing that the initial concentration of phosphoric acid
within a pool is 0.3mg. L-¹, and considering the equilibrium constants and the equilibrium expressions placed in the figure, determine the concentration of all specific species in the solution.
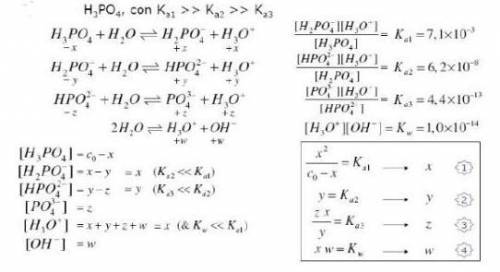

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:10
Nitrogen (n), phosphorus (p), and potassium (k) are the main nutrients in plant fertilizers. according to an industry convention, the numbers on the label refer to the mass percents of n, p2o5, and k2o, in that order. calculate the n: p: k ratio of a 30: 10: 10 fertilizer in terms of moles of each element, and express it as x: y: 1.0.
Answers: 1

Chemistry, 22.06.2019 02:00
In which of these cases are the two wave points considered to be in phase with each other?
Answers: 1

Chemistry, 22.06.2019 08:00
If 90.0 grams of ethane reacted with excess chlorine,how many grams of dicarbon hexachloride would form
Answers: 1

You know the right answer?
Questions


English, 28.01.2021 20:40

Social Studies, 28.01.2021 20:40


Biology, 28.01.2021 20:40

Mathematics, 28.01.2021 20:40

English, 28.01.2021 20:40


Mathematics, 28.01.2021 20:40

Mathematics, 28.01.2021 20:40

Mathematics, 28.01.2021 20:40



Mathematics, 28.01.2021 20:40



Chemistry, 28.01.2021 20:40

Mathematics, 28.01.2021 20:40


Mathematics, 28.01.2021 20:40



