Answers: 3


Another question on Chemistry

Chemistry, 20.06.2019 18:04
The volume of a sphere is given by v= (4/3) pi r cubed, where r is the radius. compute the volume of a sphere with a radius of 117pm. state your answer in units of cubed.
Answers: 1

Chemistry, 22.06.2019 19:30
Which liquid (h2o, h2o + soap, or h2o + salt) has the strongest cohesion and adhesion? (need now plz)
Answers: 1

Chemistry, 23.06.2019 02:00
What is the source of continuous heat and energy that we receive from the sun
Answers: 2

Chemistry, 23.06.2019 05:30
The term gas is limited to those substances that exist in the gaseous state at
Answers: 1
You know the right answer?
Chem-S: Unit 11 Test Level
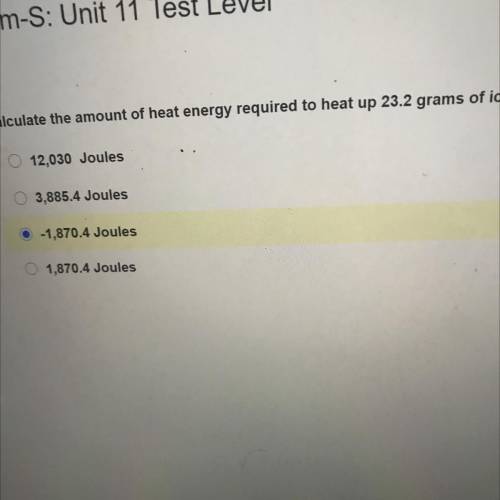
Calculate the amount of heat energy required to heat up 23.2 grams of i...
Questions




Mathematics, 12.03.2021 22:50

Mathematics, 12.03.2021 22:50

Mathematics, 12.03.2021 22:50

Mathematics, 12.03.2021 22:50


Mathematics, 12.03.2021 22:50




Mathematics, 12.03.2021 22:50

History, 12.03.2021 22:50




Spanish, 12.03.2021 22:50

Mathematics, 12.03.2021 22:50

Mathematics, 12.03.2021 22:50