
Chemistry, 22.04.2021 01:00 mivantechenko9751
A solution is prepared by dissolving 0.23 mol of chloroacetic acid and 0.27 mol of sodium chloroacetate in water sufficient to yield 1.00 L of solution. The addition of 0.05 mol of HCl to this buffer solution causes the pH to drop slightly. The pH does not decrease drastically because the HCl reacts with the _ present in the buffer solution. The 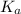 of chloroacetic acid is 1.36 x 10⁻³.
a. H₂O
of chloroacetic acid is 1.36 x 10⁻³.
a. H₂O
b. Na⁺
c. chloroacetate ion
d. chloroacetic acid
e. This is a buffer solution: the pH does not change upon addition of acid or base.

Answers: 3


Another question on Chemistry



Chemistry, 22.06.2019 00:30
Used the balanced equation 2h2+ o2 - -> 2h2o. if you have 7.2 grams of o2 , how many grams of h2o can you produce ?
Answers: 2

Chemistry, 22.06.2019 02:30
Margaret wants to make an orange flavored drink by stirring powdered drink mix into a glass of water. she doesn't like drinks that have small clumps of powdered solid in them, so she wants the drink to be a perfect solution. what factors should margaret not consider when deciding how much powder to add to her glass of water?
Answers: 3
You know the right answer?
A solution is prepared by dissolving 0.23 mol of chloroacetic acid and 0.27 mol of sodium chloroacet...
Questions









Mathematics, 14.07.2020 22:01




Mathematics, 14.07.2020 22:01

Mathematics, 14.07.2020 22:01


History, 14.07.2020 22:01


English, 14.07.2020 22:01




