
Chemistry, 22.04.2021 01:00 Jadalamanna
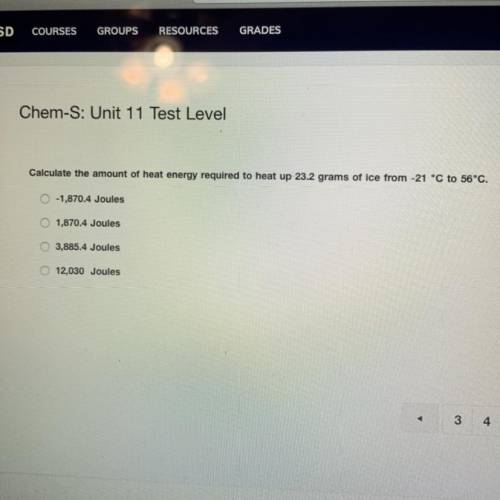
calculate the amount of heat energy required to heat up 23.2 grams of ice from -21° C to 56° C ** please show your work**

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 13:30
If a 60-g object has a volume of 30 cm3, what is its density? 2 g/cm3 0.5 cm3/g 1800 g * cm3 none of the above
Answers: 3



Chemistry, 22.06.2019 04:30
In which phase(s) do the molecules take the shape of the container?
Answers: 1
You know the right answer?
calculate the amount of heat energy required to heat up 23.2 grams of ice from -21° C to 56° C ** pl...
Questions





History, 23.04.2021 20:30


Geography, 23.04.2021 20:30

Mathematics, 23.04.2021 20:30

Mathematics, 23.04.2021 20:30




English, 23.04.2021 20:30



Mathematics, 23.04.2021 20:30




Mathematics, 23.04.2021 20:30



