2 H202 - 2 H2O + O2

Chemistry, 22.04.2021 04:40 cjasmine626
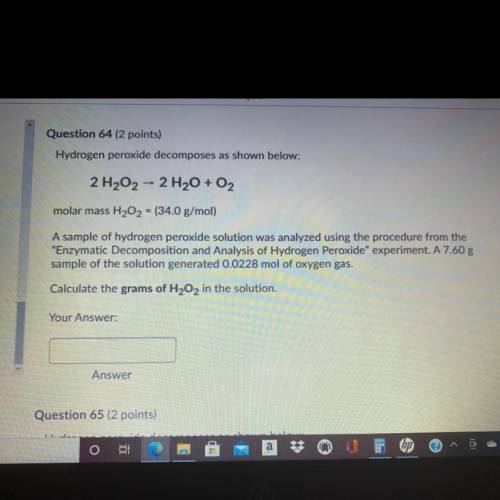
Question 64 (2 points)
Hydrogen peroxide decomposes as shown below:
2 H202 - 2 H2O + O2
molar mass H202 = (34.0 g/mol)
A sample of hydrogen peroxide solution was analyzed using the procedure from the
"Enzymatic Decomposition and Analysis of Hydrogen peroxide" experiment. A 7.60 g
sample of the solution generated 0.0228 mol of oxygen gas.
Calculate the grams of H2O2 in the solution.
Your
Answer

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1

Chemistry, 22.06.2019 18:00
How does climate change cause the ocean's thermohaline current to slow down?
Answers: 3

Chemistry, 22.06.2019 21:40
A5 mole sample of liquid acetone is converted to a gas at 75.0°c. if 628 j are required to raise the temperature of the liquid to the boiling point, 15.600 kj are required to evaporate the liquid, and 712 j are required to raise the final temperature to 75.0°c, what is the total energy required for the conversion?
Answers: 3

Chemistry, 23.06.2019 01:00
Which statement best describes isomers? a. isomers are alcohols that have the same functional group. b. isomers have at least one carbon-carbon double bond. c. isomers have the same molecular formula but different structural properties.
Answers: 1
You know the right answer?
Question 64 (2 points)
Hydrogen peroxide decomposes as shown below:
2 H202 - 2 H2O + O2
2 H202 - 2 H2O + O2
Questions


Mathematics, 06.11.2020 19:30

Chemistry, 06.11.2020 19:30



Chemistry, 06.11.2020 19:30

Mathematics, 06.11.2020 19:30




English, 06.11.2020 19:30

Computers and Technology, 06.11.2020 19:30

Engineering, 06.11.2020 19:30

Mathematics, 06.11.2020 19:30


History, 06.11.2020 19:30


Social Studies, 06.11.2020 19:30




