
Chemistry, 22.04.2021 14:00 alyahmarie00
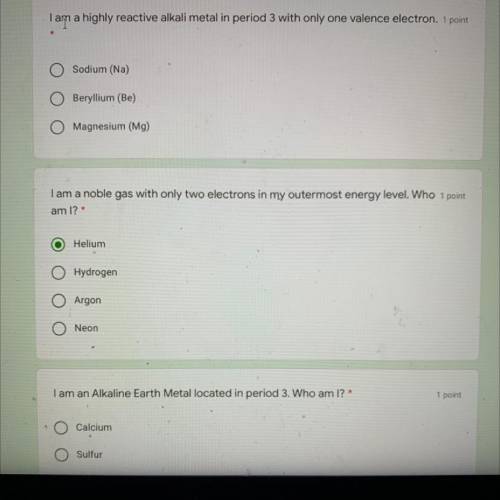
Tam a highly reactive alkali metal in period 3 with only one valence electron
O Sodium (Na)
Beryllium (Be)
Magnesium (Mg)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 14:30
Someone, part 1: which compound does c represent? part 2: name a process that could release this compound into the air. part 3: explain how the elements that form it are conserved during the carbon cycle. use complete sentences to explain your answer. justify how this compound was created from a recycling of carbon in the carbon cycle. use complete sentences to explain your answer.
Answers: 1

Chemistry, 22.06.2019 06:30
Suppose a lab group reports a ppercent yield of sand of 105. is it really possible to collect more sand than was originally represented? what is the possible explanation for the extra product?
Answers: 2

Chemistry, 22.06.2019 15:00
Many ionic compounds and a few highly polar covalent compounds are because they completely ionize in water to create a solution filled with charged ions that can conduct an electric current.
Answers: 1

You know the right answer?
Tam a highly reactive alkali metal in period 3 with only one valence electron
O Sodium (Na)
...
...
Questions





Geography, 23.11.2020 06:10


Biology, 23.11.2020 06:10


History, 23.11.2020 06:10

Chemistry, 23.11.2020 06:10

History, 23.11.2020 06:10

Mathematics, 23.11.2020 06:10


Mathematics, 23.11.2020 06:10

Chemistry, 23.11.2020 06:10




English, 23.11.2020 06:10

Biology, 23.11.2020 06:10



