
Chemistry, 22.04.2021 22:30 Samonerob2002
A solution of carbonic acid, H2CO3(aq), is at equilibrium. How would the system change if more carbonic acid were added to the solution?
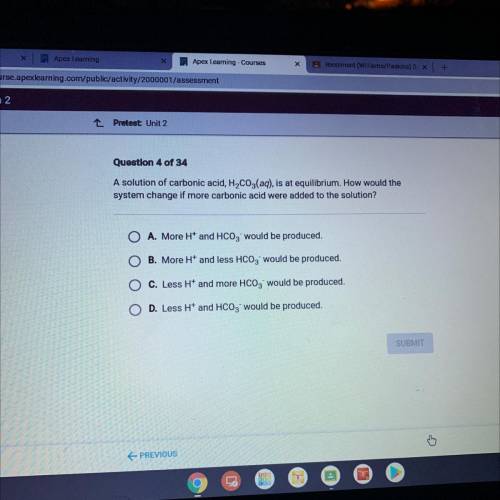

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:00
How do scientists think that gravity affected the formation of our solar system?
Answers: 1

Chemistry, 22.06.2019 05:30
Which of the following two events occur to create a sea breeze? select all that apply. warm air rises on the ocean and moves toward the land to cool warm air rises on land and moves toward the ocean to cool cool air moves from the ocean to be warmed by the land cool air moves from the land to be warmed by the ocean
Answers: 3

Chemistry, 22.06.2019 13:10
Which electron configuration represents the electrons in an atom of sodium in the ground state at stp
Answers: 1

Chemistry, 22.06.2019 19:50
Which sentence from holes contains an implied personality trait? stanley and his parents had tried to pretend that he was just going away to camp for a while, just like rich kids do. he'd just been in the wrong place at the wrong time. stanley felt somewhat dazed as the guard unlocked his handcuffs and led him off the bus. stanley nodded to show he understood
Answers: 3
You know the right answer?
A solution of carbonic acid, H2CO3(aq), is at equilibrium. How would the
system change if more car...
Questions






Computers and Technology, 02.08.2019 02:00




Mathematics, 02.08.2019 02:00


World Languages, 02.08.2019 02:00






Mathematics, 02.08.2019 02:00





