
Chemistry, 23.04.2021 01:00 nicolecoulthard
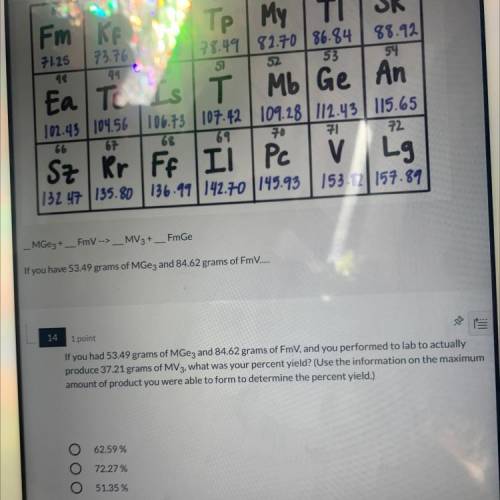
If you had 53.49 grams of MGez and 84.62 grams of FmV, and you performed to lab to actually produce 37.21 grams of MV3, what was your percent yield?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:30
How would the number of moles (n) of o2 change if the atmospheric pressure doubled but all other variables stayed the same
Answers: 2

Chemistry, 22.06.2019 09:30
Melissa is interested in her family tree and how her family has changed over its many generations. melissa probably more closely resembles
Answers: 2


Chemistry, 23.06.2019 06:00
Which factor is likely to impact the possible number of compounds? presence of unlimited number of elements in the periodic table the inability of atoms to align perfectly with other atoms the ability of all elements to react with every other element all elements being equally reactive
Answers: 2
You know the right answer?
If you had 53.49 grams of MGez and 84.62 grams of FmV, and you performed to lab to actually
produ...
Questions





Mathematics, 27.04.2020 03:06


Mathematics, 27.04.2020 03:06


Mathematics, 27.04.2020 03:06

Social Studies, 27.04.2020 03:06



Mathematics, 27.04.2020 03:06

Mathematics, 27.04.2020 03:06

History, 27.04.2020 03:06

Computers and Technology, 27.04.2020 03:06




Mathematics, 27.04.2020 03:06




