
Chemistry, 23.04.2021 06:50 bubbles173883
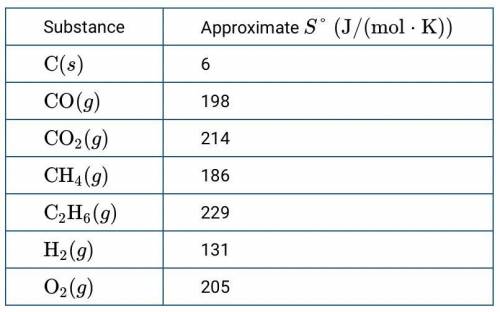
The table above provides approximate S° values for several substances. Based on the information, which of the following reactions has the largest increase in entropy, ΔSo
?
A) 2C(s)+O2(g)→2CO(g)
2 C solid plus O 2 gas react to produce 2 C O gas
B) C(s)+O2(g)→CO2(g)
C solid plus O 2 gas react to produce C O 2 gas
C) C(s)+2H2(g)→CH4(g)
C solid plus 2 H 2 gas react to produce C H 4 gas
D) D2C(s)+3H2(g)→C2H6(g)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:30
The is a particle with one unit of positive charge a. proton b. positron c. electron d. nucleus awnser quick it is a important science test!
Answers: 2

Chemistry, 22.06.2019 11:50
Acompound has a molecular weight of 12.124 atomic mass units and the empirical formula c3h40. what is the molecular formula of the compound?
Answers: 3

Chemistry, 22.06.2019 16:20
When water dissolves sugar, which process is not involved? o dissociation o hydration o surface area of the solute increases sa
Answers: 1

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium
Answers: 1
You know the right answer?
The table above provides approximate S° values for several substances. Based on the information, whi...
Questions

Social Studies, 29.01.2021 20:20



Mathematics, 29.01.2021 20:20



Mathematics, 29.01.2021 20:20

English, 29.01.2021 20:20

SAT, 29.01.2021 20:20



English, 29.01.2021 20:20





Physics, 29.01.2021 20:20

Mathematics, 29.01.2021 20:20

Mathematics, 29.01.2021 20:20



