
Chemistry, 23.04.2021 17:10 esuqugip9498
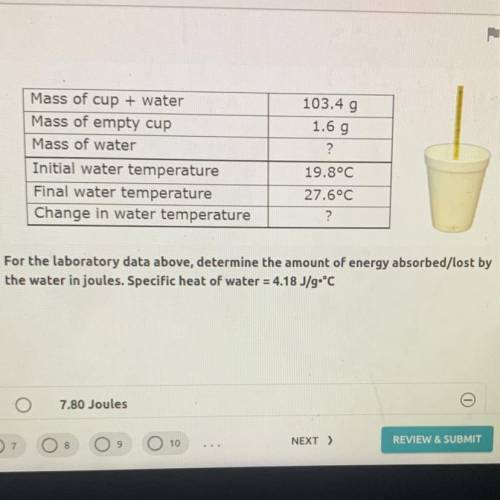
For the laboratory data above, determine the amount of energy absorbed/lost by the water in joules. Specific heat of water = 4.18 J/g•°C

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 16:10
Given the following equation: 2a1 + 3mgcl2 --> 2alcl3 + 3mg how many moles of aluminum chloride are produced from 2.5 moles of magnesium chloride?
Answers: 1

Chemistry, 23.06.2019 00:00
This statement about matter and its behavior is best classified as a
Answers: 1

Chemistry, 23.06.2019 00:50
The chemical formula for emerald is be3al2(sio3)6.an emerald can be decided as
Answers: 3

Chemistry, 23.06.2019 07:00
4. glenn andrews recently bought a motorcycle for $3,950. if he had to pay 6% sales tax on the bike, what was the total cost of the motorcycle?
Answers: 1
You know the right answer?
For the laboratory data above, determine the amount of energy absorbed/lost by
the water in joules...
Questions

Mathematics, 11.08.2021 14:00

English, 11.08.2021 14:00

Social Studies, 11.08.2021 14:00




English, 11.08.2021 14:00


Mathematics, 11.08.2021 14:00


Biology, 11.08.2021 14:00

English, 11.08.2021 14:00



Mathematics, 11.08.2021 14:00

English, 11.08.2021 14:00

English, 11.08.2021 14:00

Social Studies, 11.08.2021 14:00


Physics, 11.08.2021 14:00



