
Chemistry, 23.04.2021 18:40 WilliamYES9164
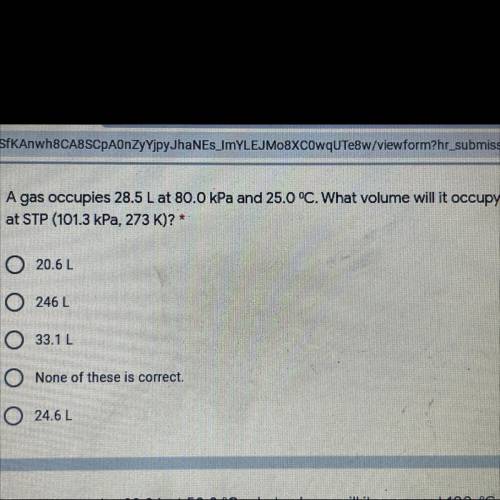
A gas occupies 28.5 L at 80.0 kPa and 25.0 °C. What volume will it occupy
at STP (101.3 kPa, 273 K)? *
A. 20.6L
B. 246 L
C. 33.12
D. None of these is correct.
E. 24.62

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Match each object to its description: a. coma of a comet b. comet tail c. oort cloud haze surrounding a nucleus created by solar wind. hypothetical sphere around the solar system
Answers: 1

Chemistry, 22.06.2019 19:30
What is the common name for the compound shown here? enter the common name of the compound shown?
Answers: 2


Chemistry, 22.06.2019 22:30
You just calculated that the heat of fusion for chloromethane is 6400 j/mol. the heat of fusion for hydrogen is 120 j/mol.? which of the following account for this difference? more than one correcta. chloromethane can absorb more energy at the same temperature. b. hydrogen has stronger intermolecular forces than chloromethane. c. hydrogen molecules can pack more closely than chloromethane molecules. d. chloromethane experiences dipole-dipole interactions. e. chloromethane has a higher molar mass than hydrogen.
Answers: 3
You know the right answer?
A gas occupies 28.5 L at 80.0 kPa and 25.0 °C. What volume will it occupy
at STP (101.3 kPa, 273...
Questions

Mathematics, 31.01.2021 02:00


Physics, 31.01.2021 02:00

History, 31.01.2021 02:00


Mathematics, 31.01.2021 02:00


Mathematics, 31.01.2021 02:00


Mathematics, 31.01.2021 02:00

History, 31.01.2021 02:00


Mathematics, 31.01.2021 02:00


Mathematics, 31.01.2021 02:00


Mathematics, 31.01.2021 02:00





