
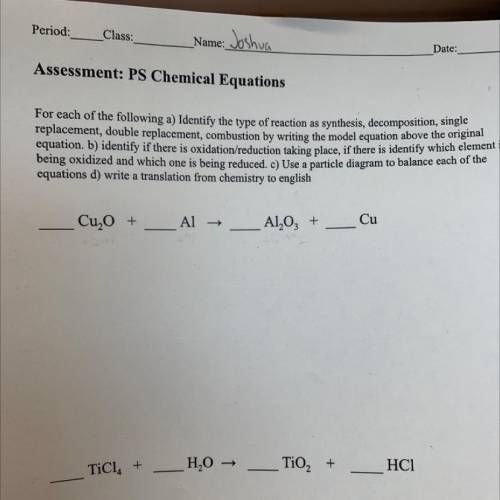
Assessment: PS Chemical Equations
For each of the following a) Identify the type of reaction as synthesis, decomposition, single
replacement, double replacement, combustion by writing the model equation above the original
equation, b) identify if there is oxidation/reduction
taking place, if there is identify which element i
being oxidized and which
one is being reduced. C) Use a particle diagram to balance each of the
equations d) write a translation from chemistry to english
Cu, O +
Al →
Al2O3 +
Cu
TiCl. +
H2O →
TiO2 +
HC1

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:30
What are the first three quantum numbers for the electrons located in subshell 2s?
Answers: 2

Chemistry, 22.06.2019 10:00
Why is the structure of molecule important to its function?
Answers: 1

Chemistry, 22.06.2019 10:00
According to the tide table below what time of day will the highest tide occur? (2 pt) the highest tide will occur at
Answers: 1

You know the right answer?
Assessment: PS Chemical Equations
For each of the following a) Identify the type of reaction as sy...
Questions


Mathematics, 22.10.2020 23:01



Chemistry, 22.10.2020 23:01


Health, 22.10.2020 23:01



Geography, 22.10.2020 23:01


Mathematics, 22.10.2020 23:01


Mathematics, 22.10.2020 23:01




Chemistry, 22.10.2020 23:01


History, 22.10.2020 23:01



